The law
Coulomb's law states that
the magnitude of the Electrostatics
force of interaction between two point charges is directly proportional
to the scalar multiplication of the magnitudes of charges and inversely
proportional to the square of the distances between them.
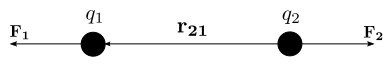
If the two charges
have the same sign, the electrostatic force between them is repulsive;
if they have different sign, the force between them is attractive.
The scalar and vector forms of the mathematical equation are
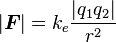 |
and |
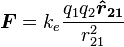 |
, respectively. |
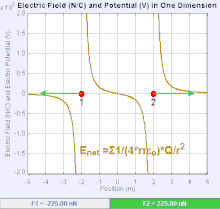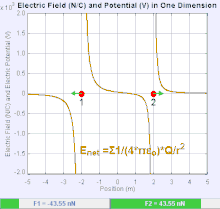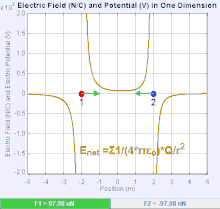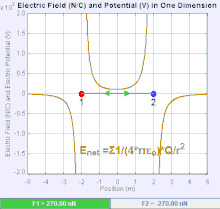
An electric field
If the two charges have the same sign, the electrostatic force between
them is repulsive; if they have different sign, the force between them
is attractive.
The magnitude of the electric field force,
E, is invertible from Coulomb's law. Since
E = F ⁄ Q it follows from the Coulomb's law that the magnitude of the electric field
E created by a single point charge
q at a certain distance
r is given by:this law is used very much in the world for many things.it can not be challenge.
 .
.
An electric field is a vector field
which associates to each point of the space the Coulomb force that will
experience a test unity charge. Given the electric field, the strength
and direction of a force
F on a
quantity charge
q in an electric field
E
is determined by the electric field. For a positive charge, the
direction of the electric field points along lines directed radially
away from the location of the point charge, while the direction is
towards for a negative charge.
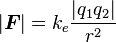
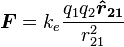
 .
.

No comments:
Post a Comment